Webinar Key Takeaways: Leveraging Blockchain in Life Sciences for Track and Trace Compliance
By Healthcare Triangle
Mar 16, 2022
 Creating end-to-end visibility in supply chain management and maintaining prescription drug traceability and integrity holds utmost importance in the pharmaceutical industry, which is currently accelerated by the mandatory compliance of the Drug Supply Chain Security Act (DSCSA).
Creating end-to-end visibility in supply chain management and maintaining prescription drug traceability and integrity holds utmost importance in the pharmaceutical industry, which is currently accelerated by the mandatory compliance of the Drug Supply Chain Security Act (DSCSA).
In a recent webinar hosted by Healthcare Triangle, industry leaders Raj Srinivas, Chief Technology Officer at Blockedge.io, and Marshall Brickeen, Global Head Life Sciences at AWS Marketplace, discussed the key strategies to stay compliant with DSCSA standards and how multi-cloud/multi-chain blockchain solutions can be deployed and managed to tackle the issues around counterfeit/expired drugs.
Pharmaceutical Counterfeiting is Significant
A joint study conducted by the European Union Intellectual Property Office (EUIPO) and the Organization for Economic Cooperation and Development (OECD) in April 2020 revealed:
- Between 2014 and 2016, pharmaceuticals were the 10th most counterfeited type of product in international trade.
- 96% of websites offering pharmaceuticals are operating illegally.
- The total value of counterfeit pharmaceuticals traded internationally in 2016 is estimated to be worth $4.4B USD.
Counterfeiting harms multiple parties, i.e., patients, providers, manufacturers, and governments across the supply chain ecosystem resulting in erosion of trust and increased cost of care. The Drug Supply Chain Security Act (DSCSA) comes into action to combat the adverse effects of pharmaceutical counterfeiting.
Track and Trace Challenges
Supply chain resiliency has become the essence as more industry leaders are investing in track and trace solutions to facilitate end-to-end visibility, and greater compliance. Some of the key challenges include:
- Outdated paper processes
- Disjointed data systems
- Lack of transparency
- No single source of truth
Blockchain Addresses Drug Supply Chain Issues
- With blockchain, you have immutable records, the production updates are documented in a single shared ledger
- Transactions are always time-stamped and up-to-date
- Complete data visibility and a single source of truth
- Regulatory compliance ensured by ledger audit trail
- Can be combined with smart technology like Internet of Things
PharmaLedger and MediLedger are trying to bring together different parties for blockchain. Traditionally, one manufacturer will create a solution and then solicit buy-in from other parts of the supply chain. But that becomes tough to manage where multiple parties are involved, so to overcome this, PharmaLedger and MediLedger had come up with a consortium on a set platform.
PharmaLedger
This is sponsored by the European Federation of Pharmaceutical Industries and Associations (EFPIA) and the EU’s Innovative Medicines Initiative (IMI) in 2020. It involves a 36-month project with 12 global pharmaceutical companies and 17 public and private entities, including legal, regulatory, academic, research, and patient representative organizations. The goal is to provide a widely trusted platform that supports the adoption of blockchain-enabled solutions for supply chains, clinical trials, and patient health records.
MediLedger Networks
It is managed by a San Francisco startup Chronicled. They conducted a pilot with leaders from 25 pharma companies, which issued a final report in 2020 that blockchain meets the 2023 DSCSA requirements for an interoperable, confidential change of ownership system within the pharma supply chain. The focus is on contracts & chargebacks and product verification for manufacturers, wholesalers, and Group Purchasing Organizations (GPOs).
DSCSA Compliance – Requirements
When did DSCSA start? In 2013 the FDA thought to outline steps to achieve interoperable, electronic tracing of products at the package level to identify and trace certain prescription drugs as they are distributed in the United States.
And then slowly progressed to the Interoperability & Saleable Returns Requirement (Deadline – November 27, 2023).
- Serialization happening at the individual drug level is one of the FDA’s requirements.
- Instead of Advanced Shipping Notice (ASN), FDA mandated Electronic Product Code Information Services (EPCIS), which reveals more specifications about the product like Product Identifier (PI) based verification – NDC, EXP, S.No., Lot No.
- Another requirement was the need to have authorized trading partners for tracking and validation (DID, W3 standard).
Why Blockchain in DSCSA – Track & Trace?
- Source of Truth (Immutable) Drug Product Information: Instantaneously shared across the ecosystem, namely manufacturers, third-party logistic providers, dispensaries.
- Source of Truth of Authorized Trading Partners: Shared for other partners in the network to verify.
- Validation of Saleable Returns: Validated electronically before re-entering the supply chain.
- Extension of Product Tracing and Validation: Applicable to all other prescription drugs (in the future) if there is a particular.
Challenges in Building and Scaling Blockchain Networks
It starts with the initial challenges (Phase 1) followed by the advanced scalability challenges (Phase 2).
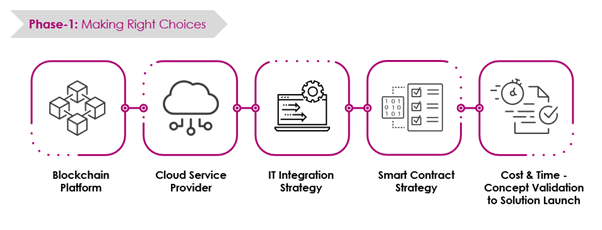
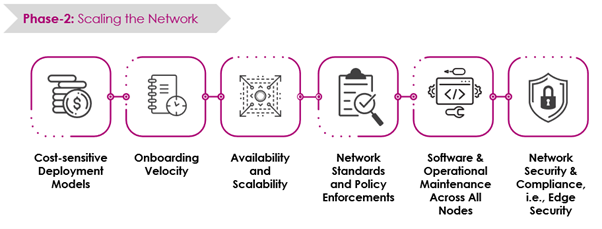
Key Aspects of Accelerating Blockchain Adoption
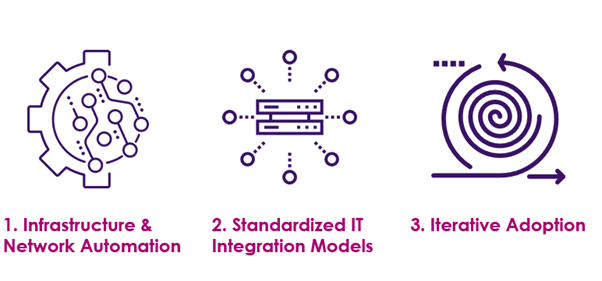
How to accelerate blockchain adoption with all these challenges and make the technology portable to all the parties. Organizations should concentrate on the following key areas for rapid blockchain adoption:
After the webinar discussion, attendees had several questions. Most of them centered around the best practices for blockchain adoption across the industry to ensure end to end verifiability and traceability of the drugs. Here are the answers to the top, most asked questions:
1. How can an organization in pharma & life sciences prepare and plan to join DSCSA network or efforts?
First, we must prepare a questionnaire to address all three aspects (business, compliance, and technical). Once the goals and objectives are defined, we need to comply, like what data we are putting out. The third part focuses on technology; it will be helpful if we try to automate some or most of the processes, like selecting cloud and blockchain. The most challenging part is the IT integration because every company has a unique system of applications. The challenge is choosing the right tools to integrate with the external world like blockchain. The adoption cannot be done overnight, do it iteratively and achieve a steady state.
2. How does AWS help customers comply with DSCSA?
AWS makes the tools or building blocks to create the solutions. One example is the Amazon blockchain engine, and another one is AWS IoT services to monitor products in transit or manufacturing. But most customers are looking to buy solutions; vendors often use AWS tools to create something more and then have it customized more out of the box and deliver those to customers.
3. Can you give some specific examples of how this prevents counterfeiting?
There are several ways; it’s all about verifying the code. This not just works for counterfeiting, but also drug recalls, so if there’s an issue, we can quickly identify where those drugs are, they need to be recalled. It’s very traceable, and those are some examples that prevent counterfeiting and help with recalls.
4. Technical wise, what are the critical steps to shift from traditional systems/internal applications to blockchain?
We are not going to build anything new for blockchain; it will be coming from the internal applications. Let’s take DSCSA as an example where you have an internal set of ERP systems that produce data, but how do we take that to blockchain; so, you will have a layer built-in which will filter out the data that is coming out, so that it could be blockchain-enabled. First, there is no need to put all the data because the mandate is only for tracking and tracing drugs. So, any PII should be filtered out.
For example, we had discussed the case pallet scenario where a lot of aggregation is happening for a particular lot to be identified. From the lot, you can have a parent-child relationship. So those kinds of scenarios where you know these events are coming through your ERP systems, and then you need to have a transformer or a payload transformer to take that and transform it into a message that is required for the blockchain. The messaging layer has to be strong because once you have the messages filled in your inbox, you should be solving it in real-time; the messages could be asynchronous or synchronous.
The API layer that is fronting this is critical because you don’t want any external parties to be hitting this layer. You must have a filtered approach with a security built-in to know only the authenticated calls are coming in through the API. And finally, there is an audit requirement; you can use Elastic search, Logstash, and Kibana or go for AWS. Depending upon the organizational requirement, the integration can be done smoothly.
5. How to tackle advanced challenges like scalability/availability/time to market?
Once you have a steady-state blockchain running, the network has partners of different types: manufacturers, third parties, logistic products, CMOs, dispensers, or pharmacies. Once we have all these different entities, it is hard to manage and maintain the network sanity. Based on the issue, each one could be in a separate network, for example, AWS, on-prem, or private cloud. With this dichotomy in view, how scalability problems would be addressed. That’s where the role of a good blockchain consulting service and a solution engineering team come into play to maintain the scalability problem. For example, if its cloud – how to do autoscaling and improve the disaster recovery processes? The process is done by hosting in different regions and peers coming in and out; if there is a need, it can be refurbished as and when required or scaled immediately and independently of the other peers in the same organization. If we implement the management techniques, scalability and availability issues can be addressed.
Due to its immutability, and traceability, blockchain offers accurate end-to-end tracking and monitoring of the drug supply chain. Now it’s time to realize the full potential of blockchain and transform your drug supply chain at scale.
No matter where you are on the blockchain journey, our experts can help you take the next step.
Drop an email to info@healthcaretriangle.com or schedule a consultation.


